第三方账号登录
 微信登录
微信登录

| 姓名 | 负责地区 | 电话 |
|---|---|---|
| 陈 |
多城市 | 多省份
|
13817358225 |
| 产品名称: | Novel Coronavirus (2019-nCoV) RT-PCR Detection Kit |
| 包装规格: | 48 tests/kit; 96 tests/kit |
| China NMPA: | China FDA certificate number: GUOXIEZHUZHUN 20203400299(国械注准20203400299) |
| CE Ref. No: | CE certification number Ref. No.:GZ8821-2020 Order. No.:GZ8776-2020 |
| Features: | Comprehensive: 3 targets (ORF1ab, N and E genes) detected in 1 tube Reliable: Internal control, UNG enzyme and dUTP were used to reduce risk of contamination and false positive results Faster results: 1.5 hours post-extraction turnaround time Sample Type:Nasopharyngeal swab/ Throat swab / Sputum/Stool Instrument: Four-channel RT-PCR instrument(FAM/JOE/ROX/CY5) |
| P I: | Product information Registration certificate: CE: Ref. No.: GZ 8821-2020 China NMPA: GUOXIEZHUZHUN 20203400299 Specification: 48 tests/kit; 96 tests/kit Sample type: Nasopharyngeal swab/ Throat swab / Sputum/Stool Sensitivity: 300 copies/mL Amplification time: 1h20min Instrument: Four-channel RT-PCR instrument(FAM/JOE/ROX/CY5) Storage & Shelf Life: -25℃~-15℃, 12 months |
| Performance: | Performance
Compared with the results of clinical diagnosis, the clinical sensitivity was 99.53%, and the clinical specificity was 97.92%.
|
| Donations: | Donations and sales The nucleic acid detection kit donated by Fosun to the Portuguese central government this time has passed the emergency approval of the State Drug Administration and the EU CE certification, and obtained the medical device product registration certificate. At present, our nucleic acid detection kits have been exported to South Korea, Portugal, Hungary, Germany, Indonesia and other countries in the form of donations or sales. |
| China NMPA: |  |
| CE: Ref.: |  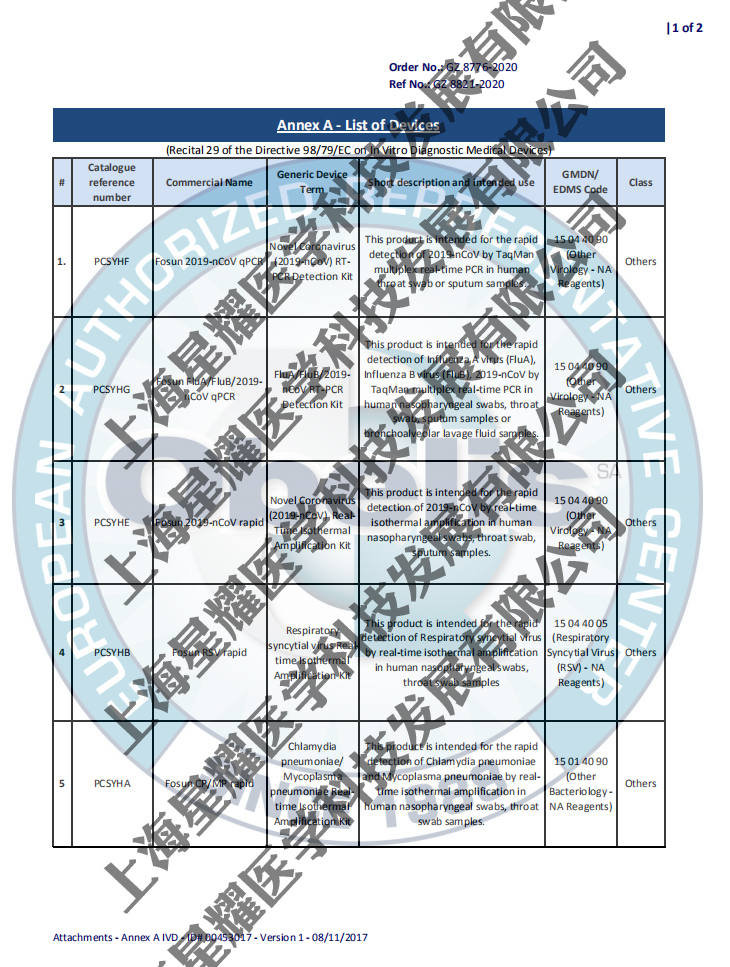  |